The claim that the composition of blood is similar to sea water is based on the assumption that life came from the ocean. The following table shows that reality is more complex. Furthermore, even if blood and sea water were similar, this wouldn’t prove one came from the other. Correlation is not causation.
Quinton claimed that in the beginning of Earth’s formation, sea water was about one third less salty. Hence, his isotonic formula. However, to my knowledge, Quinton has not fully supported this claim. On the other hand, there appears to be a consensus among the geology and experts of today that the sale level has not changed for the last 1.5 billion years.
That there are similatities between blood and ocean water is undeniable. But the hypothesis that all of Earthly Life came from the Ocean is still a hypothesis awaiting to be better proved.
Quinton postulated that all of Life came from an Ocean that was one third less salty. Hence, by injecting salt water that is diluted to match the alleged primitive ocean, one could cure many diseases and restore the body.
There is some experimentation that proves that pure ocean water can help to alleviate and sometimes even cure diseases. (See Exhibit. ). But there would need to be human clinical trial to better ascertain this theory.
As for the three dogs Quinton used to show that ocean water could replace blood, these experiments would also need to be repeated, first with rodents, then larger mammals and humans.
A scant look at Clinical trials have not shown any experimentation in this field. This type of experimentation would not be that expensive to carry on. Because none exist, we have to be cautious and not embrace Quiton as a cure all. We are open to it’s healing effect. But more studies and evidence are warranted.
This idea is often propagated as a fact by science teachers and evolutionists because they use it as a ‘proof’ for evolution. If our bodies contain the same proportion of mineral salts as the sea, they then infer that life evolved from the sea.
Sounds very scientific. Sounds very convincing. But….. is it a real proof of evolution? Or is it just a conjectured hypothesis?
BACKGROUND
In 1903 Macallum (3) (4) proposed that life originated in the sea because the relative concentrations of sodium, potassium, and calcium were in very similar proportions to those found in seawater. The actual concentrations are less than seawater.
One textbook says that, “Macallum’s theory seems, on the whole, well founded, and certainly the resemblance of the salt content of the blood of widely differing species of animals to that of sea water is striking” (5). [emphasis mine]
DATA
The following table is the type that is usually displayed as supporting proof of the theory.
Table 1 – Concentration of mineral salt ions in various living things (6)
|
Substance |
Sodium ions
Potassium ions
Calcium ions
Sea water
100
3.6
3.9
Tissue of a jellyfish
100
5.2
4.1
Serum of a lobster
100
3.7
4.9
Blood serum of a dog
100
6.9
2.5
(percentage concentration of sodium set at 100 in each substance for comparison sake)
The remainder of the data tables appear on pages 4 & 5.
ANALYSIS
(1) Does the concentration in the whole human body match seawater?
Sodium
Potassium
Calcium
Magnesium
Chlorine
Sea Water
100
3.6
3.9
12.1
179.7
Human Body
100
133.3
1333.3
33.3
100.0
Answer – NO (see table 3 & 5)
(2) Does the concentration in human body fluid match seawater?
|
Sodium |
Potassium |
Calcium |
Magnesium |
Chlorine |
|
| Sea Water |
100 |
3.6 |
3.9 |
12.1 |
179.7 |
| Human Body |
100 |
3.5 |
1.7 |
0.8 |
71.0 |
Answer – NO (see table 2 & 3)
(3) Does the concentration in human blood plasma match seawater?
|
Sodium |
Potassium |
Calcium |
Magnesium |
Chlorine |
|
| Sea Water |
100 |
3.6 |
3.9 |
12.1 |
179.7 |
| Human Body |
100 |
6.2 |
3.2 |
0.7 |
115.5 |
Answer – NO (see table 3 & 4)
(4) Do all the human mineral salt concentrations match seawater?
All major salts vary, especially magnesium, chlorine and phosphorous. There is considerable difference with iodine, sulphur, iron, copper and manganese.
Answer – NO (see table 3 & 4)
(5) Does the concentration in other animal bodies match seawater?
Jellyfish, lobster and crayfish do, as they live in water. The others all differ. The bee, beetle and clam are extremely different.
Answer – A few do, but many don’t. (see table 2)
(6) Does the correlation relate to the evolution of the animal?
Steven Austin and Russell Humphrey’s data in the graph below indicates that there was no salt in the sea over 62 million years ago. Beyond that time the body of water we now call the oceans would have been fresh water. [See my lecture notes #27 “Are the Oceans Old Enough to have Spawned Life?”].
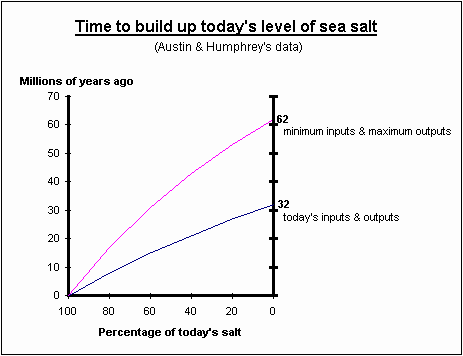
(after Figure A) (8)
Table 6 shows that animals were supposed to have evolved from the sea some 400 million years ago. At this time the sea should have been fresh water. Therefore the salt concentrations do not seem related to the time of an animal’s evolution in the sea.
Table 6 – Evolutionary origins of various animals (7)
|
ANIMAL TYPE |
EVOLVED WHEN???
Mammals
64 million years ago
Birds
182 million years ago
Reptiles
281 million years ago
Insects
311 million years ago
First land animals
426 million years ago
Bony fish
500 million years ago
Invertebrates
600 million years ago
Answer – NO
DISCUSSION
An examination of the data shows that a firm, predictable, mathematical relationship between sea water and body salts does not exist. There is too much inconsistent variation.
Many evolutionists justify the theoretical relationship by explaining away the variations. They say that it reflects the concentrations in the seawater at the time during animal evolution when their ancestors first evolved the ability to regulate salt concentrations using excretory organs – eg kidney. Macallum, like others, “suggests the very plausible idea“ (9) that during the Cambrian period when land animals are supposed to have evolved, the ocean had less salt than today.
The magnesium concentration differs markedly from the other salts, and is rationalized. “This might be explained“ (10), writes Macallum, because magnesium is not used by plants and animals as much as the other minerals, so its concentration has built up in sea water over the millions of years.
Many evolutionists believe that the variation of salts from the sea’s proportions is an indication that the animals have continued to evolve, adapting the mineral composition to the changes in the environment. This is clutching at straws. It would be just as valid to say that the differences and the similarities were all due to adaptation – independent of evolution from the sea.
When scientists believe evolution to be absolutely true, all other data is interpreted to fit in with this presupposition. One author warns about making such evolutionary inferences from this data – “We should not make the mistake of exaggerating the similarities of these fluids.” (11) Another author informs us that this theory was refuted long ago (12).
CONCLUSION
The mineral salt concentration in the tissues of living things should not be used as proof of evolution.
DETAILED TABLES
Table 2 – Percentage concentration of mineral salt ions in various living things (13)
|
Item |
Sodium ions |
Potassium ions |
Calcium ions |
Magnesium ions |
Chlorine ions |
| MARINE ANIMALS | |||||
| Jellyfish body fluid |
100 |
2.3 |
2.1 |
11.2 |
122.0 |
|
454.0 |
10.2 |
9.7 |
51.0 |
554.0 |
|
| Lobster body fluid |
100 |
2.1 |
3.3 |
1.4 |
99.6 |
|
472.0 |
10.0 |
15.6 |
6.8 |
470.0 |
|
| Sea urchin body fluid |
100 |
2.2 |
2.2 |
11.3 |
117.6 |
|
444.0 |
9.6 |
9.9 |
50.2 |
522.0 |
|
| Annelid worm body |
100 |
2.6 |
2.2 |
11.3 |
118.0 |
| fluid |
456.0 |
12.3 |
10.1 |
51.7 |
538.0 |
| Crab body fluid |
100 |
2.6 |
3.7 |
5.0 |
112.0 |
|
468.0 |
12.1 |
17.5 |
23.6 |
524.0 |
|
| FRESH WATER ANIMALS | |||||
| Clam body fluid |
100 |
2.1 |
79.1 |
2.1 |
86.3 |
|
13.9 |
0.3 |
11.0 |
0.3 |
12.0 |
|
| Crayfish body fluid |
100 |
2.7 |
5.5 |
2.9 |
95.2 |
|
146.0 |
3.9 |
8.1 |
4.3 |
139.0 |
|
| LAND ANIMALS | |||||
| Cockroach body fluid |
100 |
4.9 |
2.5 |
3.5 |
89.4 |
|
161.0 |
7.9 |
4.0 |
5.6 |
144.0 |
|
| Bee body fluid |
100 |
281.8 |
163.6 |
191.0 |
– |
|
11.0 |
31.0 |
18.0 |
21.0 |
? |
|
| Beetle body fluid |
100 |
50.0 |
80.0 |
195.0 |
95.0 |
|
20.0 |
10.0 |
16.0 |
39.0 |
19.0 |
|
| Chicken body fluid |
100 |
3.8 |
3.6 |
1.5 |
79.2 |
|
154.0 |
6.0 |
5.6 |
2.3 |
122.0 |
|
| Dog body fluid |
100 |
2.9 |
3.5 |
1.2 |
70.7 |
|
150.0 |
4.4 |
5.3 |
1.8 |
106.0 |
|
| Human body fluid |
100 |
3.5 |
1.7 |
0.8 |
71.0 |
|
145.0 |
5.1 |
2.5 |
1.2 |
103.0 |
|
(1st row – percentage concentration of sodium set at 100 in each fluid for comparison sake; 2nd row – amount in millimoles/Lt)
Table 3 – Mineral composition of sea water (14)
|
Component |
Concentration (g/kg) |
Percentage of Minerals |
Mineral Ratio |
| Water |
965.518 |
– |
|
| Sodium |
10.556 |
30.6% |
100 |
| Chloride |
18.980 |
55.0% |
179.7 |
| Potassium |
0.380 |
1.1% |
3.6 |
| Calcium |
0.400 |
1.2% |
3.9 |
| Magnesium |
1.272 |
3.7% |
12.1 |
| Sulfate |
2.649 |
7.7% |
25.2 |
| Phosphate |
N/A |
– |
|
| Phosphorous |
<0.0001 (15) |
0.1 ppm |
|
| Iron |
trace |
trace |
|
| Copper |
trace |
trace |
|
| Manganese |
trace |
trace |
|
| Iodine |
0.00005 (16) |
0.05 ppm |
Table 4 – Mineral composition of human plasma (17)
|
Component |
Concentration (g/100ml) |
Percentage of Minerals
Mineral
Ratio
Water
90-93
Sodium
0.320
43.9%
100.0
Chloride
0.370
50.7%
115.5
Potassium
0.020
2.7%
6.2
Calcium
0.010
1.4%
3.2
Magnesium
0.0025
0.3%
0.7
Sulfate
0.003
0.4%
0.9
Phosphate
0.003
0.4%
0.9
Table 5 – Mineral composition of some animal bodies
|
Mineral |
||
Human body (18)
Animal body (19)
concentration
ratio
concentration
ratio
Sodium
0.15%
100.0
0.16%
100.0
Chlorine
0.15%
100.0
0.11%
68.8
Potassium
0.20%
133.3
0.20%
125.0
Calcium
2.00%
1333.3
1.50%
937.5
Magnesium
0.05%
33.3
0.04%
25.0
Sulfur
0.25%
166.7
0.15%
93.8
Phosphorous
1.10%
733.3
1.00%
625.0
Iron
40 ppm
20-80 ppm
Copper
1.5 ppm
1-5 ppm
Manganese
1.3ppm
0.2-0.5 ppm
Iodine
0.4 ppm
0.3-0.6 ppm
Zinc
–
10-50 ppm
cobalt
–
0.02-0.10 ppm
(concentration in % wet weight)
REFERENCES
(1) The Canberra Times, Canberra, Australia, 22/9/98, p:7
(2) The Canberra Times, Canberra, Australia, 22/9/98, Techno lift-out section, p:6
(3) A.B. Macallum, “On the Inorganic Composition of the Medusae, Aurelia flavidula and Cyanea artica”, The Journal of Physiology (London), Vol. 29, 1903 p:214
(4) A.B. Macallum, “The Paleochemistry of the Body Fluids and Tissues”, Physiol. Revs., Vol. 6, 1926 p:316
(5) P.H. Mitchell, “A Textbook of General Physiology” (5th edition – Kogakusha Co. Ltd, Tokyo), McGraw-Hill Book Co: New York, 1956 p:313
(6) P.H. Mitchell, “A Textbook of General Physiology” (5th edition – Kogakusha Co. Ltd, Tokyo), McGraw-Hill Book Co: New York, 1956 p:312
(7) J.D. Morris, “The Young Earth”, Master Books: Colorado Springs (USA), 1994 p:8
(8) S.A. Austin & D.R. Humphreys, “The Sea’s Missing Salt: A Dilemma for Evolutionists”, Proceedings of the Second International Conference on Creationism, Vol. 2, 1991 p:33
(9) P.H. Mitchell, “A Textbook of General Physiology” (5th edition – Kogakusha Co. Ltd, Tokyo), McGraw-Hill Book Co: New York, 1956 p:313
(10) P.H. Mitchell, “A Textbook of General Physiology” (5th edition – Kogakusha Co. Ltd, Tokyo), McGraw-Hill Book Co: New York, 1956 p:313
(11) W.T. Keeton, “Biological Science”, W.W. Norton & Co: New York, 1967 p:271
(12) I.T. Taylor, “In the Minds of Men: Darwin and the New World Order” (3rd ed), TFE Publishing: Toronto, 1992 p:291
(13) W.T. Keeton, “Biological Science”, W.W. Norton & Co: New York, 1967 p:272
(14) “The New Encyclopedia Britannica” (15th edition), Vol. 25, Encyclopedia Britannica Inc: Chicago, 1989 p:126
(15) Australian Academy of Science, “Biological Science: The web of life” (second ed.), Australian Academy of Science: Canberra (Aust), 1973 p:150
(16) Australian Academy of Science, “Biological Science: The web of life” (second ed.), Australian Academy of Science: Canberra (Aust), 1973 p:150
(17) “The New Encyclopedia Britannica” (15th edition), Vol. 19, Encyclopedia Britannica Inc: Chicago, 1989 p:13
(18) “The New Encyclopedia Britannica” (15th edition), Vol. 25, Encyclopedia Britannica Inc: Chicago, 1989 p:50
(19) P. McDonald, R.A. Edwards & J.F.D. Greenhalgh “Animal Nutrition”, Oliver & Boyd Ltd: Edinburgh (Scotland), 1969 p:76
The information in the chart was taken from:
- C.A. Burtis and E.R. Ashwood, Clinical Chemistry. Philadelphia: W.B. Saunders Company, 1994 edition.
- R.C. Baselt and R.H. Cravey, Disposition of toxic drugs and chemicals in Man. Chicago: Year Book Medical Publ., 1989 edition.
- The New Encyclopædia Britannica, 15:925, 1992, 15th ed.
| Element | Blood | Seawater |
|---|---|---|
| Sodium | 3220 mg/liter | 10800 |
| Chlorine | 3650 | 19400 |
| Potassium | 200 | 392 |
| Calcium | 50 | 411 |
| Magnesium | 27 | 1290 |
| Phosphorus | 36 | 0.09 |
| Iron | 1 | 0.004 |
| Copper | 1 | 0.001 |
| Zinc | 1.1 | 0.005 |
| Chromium | 1.1 | 0.0002 |
| Bromine | 4 | 67 |
| Fluorine | 0.1 | 1.3 |
| Boron | 1 | 5 |
| Selenium | 0.9 | 0.0001 |
MINERAL MAKEUP OF SEAWATER
In order of most to least:
| ELEMENT | MOLECULAR WEIGHT | PPM IN SEAWATER | MOLAR CONCENTRATION |
| Chloride | 35.4 | 18980 | 0.536158 |
| Sodium | 23 | 10561 | 0.459174 |
| Magnesium | 24.3 | 1272 | 0.052346 |
| Sulfur | 32 | 884 | 0.027625 |
| Calcium | 40 | 400 | 0.01 |
| Potassium | 39.1 | 380 | 0.009719 |
| Bromine | 79.9 | 65 | 0.000814 |
| Carbon(inorganic) | 12 | 28 | 0.002333 |
| Strontium | 87.6 | 13 | 0.000148 |
| Boron | 10.8 | 4.6 | 0.000426 |
| Silicon | 28.1 | 4 | 0.000142 |
| Carbon (organic) | 12 | 3 | 0.00025 |
| Aluminum | 27 | 1.9 | 0.00007 |
| Fluorine | 19 | 1.4 | 0.000074 |
| N as nitrate | 14 | 0.7 | 0.00005 |
| Nitrogen (organic) | 14 | 0.2 | 0.000014 |
| Rubidium | 85 | 0.2 | 0.0000024 |
| Lithium | 6.9 | 0.1 | 0.000015 |
| P as Phosphate | 31 | 0.1 | 0.0000032 |
| Copper | 63.5 | 0.09 | 0.0000014 |
| Barium | 137 | 0.05 | 0.00000037 |
| Iodine | 126.9 | 0.05 | 0.00000039 |
| N as nitrite | 14 | 0.05 | 0.0000036 |
| N as ammonia | 14 | 0.05 | 0.0000036 |
| Arsenic | 74.9 | 0.024 | 0.00000032 |
| Iron | 55.8 | 0.02 | 0.00000036 |
| P as organic | 31 | 0.016 | 0.00000052 |
| Zinc | 65.4 | 0.014 | 0.00000021 |
| Manganese | 54.9 | 0.01 | 0.00000018 |
| Lead | 207.2 | 0.005 | 0.000000024 |
| Selenium | 79 | 0.004 | 0.000000051 |
| Tin | 118.7 | 0.003 | 0.000000025 |
| Cesium | 132.9 | 0.002 | 0.000000015 |
| Molybdenum | 95.9 | 0.002 | 0.000000021 |
| Uranium | 238 | 0.0016 | 0.0000000067 |
| Gallium | 69.7 | 0.0005 | 0.0000000072 |
| Nickel | 58.7 | 0.0005 | 0.0000000085 |
| Thorium | 232 | 0.0005 | 0.0000000022 |
| Cerium | 140 | 0.0004 | 0.0000000029 |
| Vanadium | 50.9 | 0.0003 | 0.0000000059 |
| Lanthanum | 139.9 | 0.0003 | 0.0000000022 |
| Yttrium | 88.9 | 0.0003 | 0.0000000034 |
| Mercury | 200.6 | 0.0003 | 0.0000000015 |
| Silver | 107.9 | 0.0003 | 0.0000000028 |
| Bismuth | 209 | 0.0002 | 0.00000000096 |
| Cobalt | 58.9 | 0.0001 | 0.0000000017 |
| Gold | 197 | 0.000008 | 0.00000000004 |
https://web.stanford.edu/group/Urchin/mineral.html
| ÉlementHydrogène H2O |
Oxygène H2O
Sodium NaCl
Chlore NaCl
Magnésium Mg
Soufre S
Potassium K
Calcium Ca
Brome BrPoidsAtomique1.00797
15.9994
22.9898
35.453
24.312
32.064
39.102
10.08 79.909ppm110,000
883,000
10,800
19,400
1,290
904
392 411 67.3ÉlementMolybdène Mo
Ruthénium Ru
Rhodium Rh
Palladium Pd
Argent Ag
Cadmium Cd
Indium In
Étain Sn
Antimoine SbPoidsAtomique0.09594
101.07
102.905
106.4
107.870
112.4
114.82
118.69
121.75ppm0.01
0.0000007
.
.
0.00028
0.00011
.
0.00081
0.00033Hélium HeLithium LiBéryllium BeBore B
Carbone C
Azote N
Fluor F
Néon Ne
Aluminium Al
Silicium Si
Phosphore P
Argon Ar
Scandium Sc
Titane Ti
Vanadium V
Chrome Cr
Manganèse Mn
Fer Fe
Cobalt Co
Nickel Ni4.00266.9399.013310.811
12.011
14.007
18.998
20.183
26.982
28.086
30.974
39.948
44.956
47.90
50.942
51.996
54.938
55.847
58.933
58.710.0000072 0.1700.00000064.45028.0
15.5
13
0.00012
0.001
2.9
0.088
0.450
<0.00000
0.001
0.0019
0.0002
0.0004
0.0034
0.00039
0.0066Tellure TeIode IXenon XeCésium Cs
Baryum Ba
Lanthane La
Cérium Ce
Praseodyme Pr
Neodyme Nd
Samarium Sm
Europium Eu
Gadolinium Gd
Terbium Tb
Dysprosium Dy
Holmium Ho
Erbium Er
Thulium Tm
Ytterbium Yb
Lutécium Lu
Hafnium Hf127.6166.904131.30132.905
137.34
138.91
140.12
140.907
144.24
150.35
151.96
157.25
158.924
162.50
164.930
167.26
168.934
173.04
174.97
178.49.0.0640.0000470.0003
0.021
0.0000029
0.0000012
0.00000064
0.0000028
0.00000045
0.0000013
0.0000007
0.00000014
0.00000091
0.00000022
0.00000087
0.00000017
0.00000082
0.00000015
<0.000008Cuivre CuZinc ZnGallium GaGermanium Ge
Arsenic As
Sélénium Se
Krypton Kr
Rubidium Rb
Strontium Sr
Yttrium Y
Zirconium Zr
Niobium Nb63.5465.3769.7272.59
74.922
78.96
83.80
85.47
87.62
88.905
91.22
92.9060.00090.0050.000030.00006
0.0026
0.0009
0.00021
0.120
8.1
0.000013
0.000026
0.000015Tantale TaTungstène WRhénium ReOsmium Os
Iridium Ir
Platine Pt
Or Au
Mercure Hg
Thallium Tl
Plomb Pb
Bismuth Bi
Thorium Th
Uranium U
Plutonium Pu180.948183.85186.2190.2
192.2
195.09
196.967
200.59
204.37
207.19
208.980
232.04
238.03
(244)<0.0000025<0.0000010.0000084.
.
.
0.000011
0.00015
.
0.00003
0.00002
0.0000004
0.0033
.
Note! ppm= parties par million = mg/litre = 0.001g/kg.
Source: Karl K Turekian: Oceans. 1968. Prentice-Hall
| Compound | Comments | In whole blood (g/cm³) | In plasma or serum (g/cm³) |
|---|---|---|---|
| Water | Solvent | 0.81-0.86 | 0.93-0.95 |
| Acetoacetate | Produced in liver | 8-40 ×10−7 | 4-43 ×10−7 |
| Acetone | product of bodyfat breakdown | 3-20 ×10−6 | |
| Acetylcholine | Neurotransmitter of the parasympathetic nervous system | 6.6-8.2 ×10−8 | |
| Adenosine triphosphate | Energy storage | ||
| total | 3.1-5.7 ×10−4 | ||
| phosphorus | 5-10 ×10−5 | ||
| Adrenocorticotrophic hormone | Stimulates the adrenal cortex | 2.5-12 ×10−11 | |
| @ 6AM, mean | 5.5 ×10−11 | ||
| @ 6AM, maximum | 12 ×10−11 | ||
| @ 6PM, mean | 3.5 ×10−11 | ||
| @ 6PM, maximum | <7.5 ×10−11 | ||
| Alanine | Amino acid | 2.7-5.5 ×10−5 | 2.4-7.6 ×10−5 |
| Albumin | Blood plasma protein | 3.5-5.0 ×10−2[1] | |
| Aluminum | 1-40 ×10−8 | 1-88 ×10−8 | |
| Aldosterone | Regulates electrolyte balance | ||
| supine | 3-10 ×10−11 | ||
| standing, male | 6-22 ×10−11 | ||
| standing, female | 5-30 ×10−11 | ||
| Amino acids | Protein building blocks | ||
| total | 3.8-5.3 ×10−4 | ||
| nitrogen | 4.6-6.8 ×10−5 | 3.0-5.5 ×10−5 | |
| alpha-Aminobutyric acid | 1-2 ×10−6 | 1-2 ×10−6 | |
| d-Aminolevulinic acid | 1.5-2.3 ×10−7 | ||
| Ammonia nitrogen | 1-2 ×10−6 | 1.0-4.9 ×10−7 | |
| cAMP | Intracellular signal transduction molecule | ||
| male | 5.6-10.9 ×10−9 | ||
| female | 3.6-8.9 ×10−9 | ||
| Androstenedione | Steroid hormone | ||
| male >18 yrs | 2-30 ×10−10 | ||
| female >18 yrs | 8-30 ×10−10 | ||
| Androsterone | Steroid hormone | 1.5 ×10−7 | |
| Angiotensin I | Angiotensin II precursor | 1.1-8.8 ×10−11 | |
| Angiotensin II | Vasoconstrictor | 1.2-3.6 ×10−11 | |
| Alpha 1-antitrypsin | Serine protease inhibitor | 7.8-20 ×10−4 | |
| Arginine | Amino acid | 6-17 ×10−6 | 1.3-3.6 ×10−5 |
| Arsenic | normal range | 2-62 ×10−9 | |
| chronic poisoning | 100-500 ×10−9 | ||
| acute poisoning | 600-9300 ×10−9 | ||
| Ascorbic acid (Vitamin C) | Important vitamin | 1-15 ×10−6 | 6-20 ×10−6 |
| Aspartic acid | Amino acid | 0-3 ×10−6 | |
| In WBCs | 2.5-4.0 ×10−4 | 9-12 ×10−6 | |
| Bicarbonate | Buffer in blood | 5-5.7 ×10−4 | |
| Bile acids | Digestive function, bilirubin excretion | 2-30 ×10−6 | 3-30 ×10−6 |
| Bilirubin | Hemoglobin metabolite | 2-14 ×10−6 | 1-10 ×10−6 |
| Biotin (Vitamin H) | Gluconeogenesis, metabolize leucine, fatty acid synthesis | 7-17 ×10−9 | 9-16 ×10−9 |
| Blood Urea Nitrogen (BUN) | 8-23 ×10−5 | ||
| Bradykinin | 7 ×10−11 | ||
| Bromide | 7-10 ×10−9 | ||
| Cadmium | normal | 1-5 ×10−9 | |
| toxic | 0.1-3 ×10−6 | ||
| Calciferol (vitamin D2) | Maintain calcium and phosphorus levels | 1.7-4.1 ×10−8 | |
| Calcitonin (CT) | Hormone | <1.0 ×10−10 | |
| Calcium | Bones, Ca2+ | ||
| ionized | 4.48-4.92 ×10−5 | 4.25-5.25 ×10−5 | |
| total | 8.4-11.5 ×10−5 | ||
| Carbon dioxide | Respiratory gas | ||
| arterial | 8.8-10.8 ×10−4 | 3.0-7.9 ×10−5 | |
| venous | 9.8-11.8 ×10−4 | 3.3-8.3 ×10−5 | |
| Carboxyhemoglobin(as HbCO) | nonsmokers | 0.5-1.5% total Hb | |
| smokers, 1-2 packs/day | 4-5% total Hb | ||
| smokers, 2 packs/day | 8-9% total Hb | ||
| toxic | >20% total Hb | ||
| lethal | >50% total Hb | ||
| Carcinoembryonic antigen | <2.5 ×10−9 | ||
| beta-Carotene | Vitamin A dimer | 3-25 ×10−7 | |
| Carotenoids | Antioxidant | 2.4-23.1 ×10−7 | |
| Cephalin | 3-11.5 ×10−4 | 0-1 ×10−4 | |
| Ceruloplasmin | 1.5-6 ×10−4 | ||
| Chloride, as NaCl | 4.5-5 ×10−3 | 3.5-3.8 ×10−3 | |
| Cholecalciferol (Vitamin D3) | 1, 25-dihydroxy | 2.5-4.5 ×10−11 | |
| 24,25-dihydroxy | 1.5 ×10−9 | ||
| 25-hydroxy | 1.4-8 ×10−8 | ||
| Cholecystokinin (pancreozymin) | Stimulates fat and protein digestion | 6.04 ×10−11 | |
| Cholesterol | Steroid lipid | ||
| LDLC | 0.5-2.0 ×10−3 | ||
| HDLC | 2.9-9.0 ×10−4 | ||
| total | 1.15-2.25 ×10−3 | 1.2-2 ×10−3 | |
| Choline, total | 1.1-3.1 ×10−4 | 3.6-3.5 ×10−4 | |
| Chorionic gonadotropin | Progesterone secretion during pregnancy | ||
| Menstrual | 0-3 ×10−11 | ||
| Pregnancy, 1st trimester | 5-3300 ×10−10 | ||
| Pregnancy, 2nd trimester | 20-1000 ×10−10 | ||
| Pregnancy, 3rd trimester | 20-50 ×10−10 | ||
| Menopausal | 3-30 ×10−11 | ||
| Citric acid | 1.3-2.5 ×10−5 | 1.6-3.2 ×10−5 | |
| Citrulline | 2-10 ×10−6 | ||
| Coagulation Factors | Fibrinogen | 1.2-1.6 ×10−3 | 2-4 ×10−3 |
| Prothrombin | 1 ×10−4 | ||
| Tissue thromboplastin | 1 ×10−6 | ||
| Proaccelerin | 5-12 ×10−6 | ||
| Proconvertin | 1 ×10−6 | ||
| Antihemophilic factor | 1 ×10−7 | ||
| Christmas factor | 4 ×10−6 | ||
| Stuart factor | 5 ×10−6 | ||
| Plasma thrmb. anteced. | 4 ×10−6 | ||
| Hageman factor | 2.9 ×10−5 | ||
| Fibrin-stabilizing factor | 1 ×10−5 | ||
| Fibrin split products | <1 ×10−5 | ||
| Fletcher factor | 5 ×10−5 | ||
| Fitzgerald factor | 7 ×10−5 | ||
| von Willebrand factor | 7 ×10−6 | ||
| Cobalamin (Vitamin B12) | Needed for nerve cells, red blood cells, and to make DNA | 6-14 ×10−10 | 1-10 ×10−10 |
| Cocarboxylase | 7-9 ×10−8 | ||
| Complement system | C1q | 5.8-7.2 ×10−5 | |
| C1r | 2.5-3.8 ×10−5 | ||
| C1s (C1 esterase) | 2.5-3.8 ×10−5 | ||
| C2 | 2.2-3.4 ×10−5 | ||
| C3( b1C-globulin) | 8-15.5 ×10−4 | ||
| factor B (C3 proactivator) | 2-4.5 ×10−4 | ||
| C4 (b1E-globulin) | 1.3-3.7 ×10−4 | ||
| C4 binding protein | 1.8-3.2 ×10−4 | ||
| C5 (b1F-globulin) | 5.1-7.7 ×10−5 | ||
| C6 | 4.8-6.4 ×10−5 | ||
| C7 | 4.9-7 ×10−5 | ||
| C8 | 4.3-6.3 ×10−5 | ||
| C9 | 4.7-6.9 ×10−5 | ||
| Properdin | 2.4-3.2 ×10−5 | ||
| Compound S | 1-3 ×10−9 | ||
| Copper | 9-15 ×10−7 | ||
| male | 7-14 ×10−7 | ||
| female | 8-15.5 ×10−7 | ||
| Corticosteroids | Steroid hormones | 1-4 ×10−6 | |
| Corticosterone | 4-20 ×10−9 | ||
| Cortisol | Inhibits CRH secretion | 3-23 ×10−8 | |
| 8 AM | 6-23 ×10−8 | ||
| 4 PM | 3-15 ×10−8 | ||
| 10 PM | ~50% of 8 AM value | ||
| C-peptide | fasting | 0.5-2.0 ×10−9 | |
| maximum | 4 ×10−9 | ||
| C-reactive protein | Plasma protein | 6.8-820 ×10−8 | |
| Creatine | Assists muscle cell energy supply | ||
| male | 1.7-5.0 ×10−6 | ||
| female | 3.5-9.3 ×10−6 | ||
| Creatinine | male | 0.8-1.5 ×10−5 | |
| female | 0.7-1.2 ×10−5 | ||
| Cyanide | nonsmokers | 4 ×10−9 | |
| smokers | 6 ×10−9 | ||
| nitroprusside therapy | 10-60 ×10−9 | ||
| toxic | >100 ×10−9 | ||
| lethal | >1000 ×10−9 | ||
| Cysteine | Amino acid | 6-12 ×10−6 | 1.8-5 ×10−5 |
| Dehydroepiandrosterone(DHEA) | Steroid hormone | ||
| aged 1–4 yrs | 0.2-0.4 ×10−9 | ||
| aged 4–8 yrs | 0.1-1.9 ×10−9 | ||
| aged 8–10 yrs | 0.2-2.9 ×10−9 | ||
| aged 10–12 yrs | 0.5-9.2 ×10−9 | ||
| aged 12–14 yrs | 0.9-20 ×10−9 | ||
| aged 14–16 yrs | 2.5-20 ×10−9 | ||
| male | 0.8-10 ×10−9 | ||
| female, premenopausal | 2.0-15 ×10−9 | ||
| DHEA sulfate | male | 1.99-3.34 ×10−6 | |
| female | |||
| newborn | 1.67-3.64 ×10−6 | ||
| pre-pubertal children | 1.0-6.0 ×10−7 | ||
| premenopausal | 8.2-33.8 ×10−7 | ||
| pregnancy | 2.3-11.7 ×10−7 | ||
| postmenopausal | 1.1-6.1 ×10−7 | ||
| 11-Deoxycortisol | 1-7 ×10−8 | ||
| Dihydrotestosterone (DHT) | male | 3-8 ×10−9 | |
| female | 1-10 ×10−10 | ||
| Diphosphoglycerate (phosphate) | 8-16 ×10−5 | ||
| DNA | The molecule of heredity | 0-1.6 ×10−5 | |
| Dopamine | Neurotransmitter | <1.36 ×10−10 | |
| Enzymes, total | <6 ×10−5 | ||
| Epidermal growth factor (EGF) | <1 ×10−11 | ||
| Epinephrine | Neurotransmitter of the sympathetic nervous system | ||
| after 15 min rest | 3.1-9.5 ×10−11 | ||
| when emitted | 3.8 ×10−9 | 2-2.5 ×10−9 | |
| Ergothioneine | 1-20 ×10−5 | ||
| Erythrocytes (#/cm³) | adult male, avg. (range) | 5.2 (4.6-6.2) ×109 | |
| adult female, avg. (range) | 4.6 (4.2-5.4) ×109 | ||
| children, varies with age | 4.5-5.1 ×109 | ||
| reticulocytes | 25-75 ×106 | ||
| Erythropoietin | adult, normal | 0.5-2.5 ×10−10 | |
| pregnant | 2.7-6.2 ×10−10 | ||
| hypoxia or anemia | 0.8-8.0 ×10−8 | ||
| Estradiol (E2) | male | 8-36 ×10−12 | |
| female, follicular(days 1-10) | 1-9 ×10−11 | ||
| female, mean | 5 ×10−11 | ||
| female, pre-fertile (days 10-12) | 10-15 ×10−11 | ||
| female, fertile (days 12-14) | 35-60 ×10−11 | ||
| female, luteal (days 15-28) | 20-40 ×10−11 | ||
| female, pregnancy | 3-70 ×10−7 | ||
| female, postmenopausal | 1-3 ×10−11 | ||
| Estriol (E3) | nonpregnant | <2 ×10−9 | |
| pregnancy, weeks 22-30 | 3-5 ×10−9 | ||
| pregnancy, weeks 32-37 | 6-11 ×10−9 | ||
| pregnancy, weeks 38-41 | 25-170 ×10−9 | ||
| Estrogen | male | 4-11.5 ×10−11 | |
| female, prepubertal | <4 ×10−11 | ||
| female, 1–10 days | 6.1-39.4 ×10−11 | ||
| female, 11–20 days | 12.2-43.7 ×10−11 | ||
| female, 21–30 days | 15.6-35 ×10−11 | ||
| female, postmenopausal | <4 ×10−11 | ||
| Estrone (E1) | male | 2.9-17 ×10−11 | |
| female, follicular | 2-15 ×10−11 | ||
| female, 1–10 days of cycle | 4.3-18 ×10−11 | ||
| female, 11–20 days of cycle | 7.5-19.6 ×10−11 | ||
| female, 20–29 days of cycle | 13.1-20.1 ×10−11 | ||
| pregnancy, weeks 22-30 | 3-5 ×10−9 | ||
| pregnancy, weeks 32-37 | 5-6 ×10−9 | ||
| pregnancy, weeks 38-41 | 7-10 ×10−9 | ||
| Ethanol | social high | 0.5 ×10−3 | |
| reduced coordination | 0.8 ×10−3 | ||
| depression of CNS | >1 ×10−3 | ||
| confusion, falling down | 2.0 ×10−3 | ||
| loss of consciousness | 3.0 ×10−3 | ||
| coma, death | >4 ×10−3 | ||
| Fatty acids | nonesterified (free) | 8-25 ×10−5 | |
| esterified | 2.5-3.9 ×10−3 | 7-20 ×10−5 | |
| total | 1.9-4.5 ×10−3 | ||
| Ferritin | male | 1.5-30 ×10−8 | |
| female | 0.9-18 ×10−8 | ||
| alpha-1-Fetoprotein | 0-2 ×10−8 | ||
| Flavin adenine dinucleotide | 8-12 ×10−8 | ||
| Fluoride | 1-4.5 ×10−7 | 1-4.5 ×10−7 | |
| Folate | 2.2-17.3 ×10−9 | ||
| in erythrocyte | 1.67-7.07 ×10−7 | ||
| Folic acid | 2.3-5.2 ×10−8 | 1.6-2 ×10−8 | |
| Fructose | 0-5 ×10−5 | 7-8 ×10−5 | |
| Furosemide glucuronide | 1-400 ×10−6 | ||
| Galactose | children | <2 ×10−4 | |
| Gastric inhibitory peptide (GIP) | <1.25-4.0 ×10−10 | ||
| Gastrin | mean | 7 ×10−11 | |
| maximum | <20 ×10−11 | ||
| Globulin | total 2.2-4 ×10−2 | ||
| alpha-1-Globulin | 1-4 ×10−3 | ||
| alpha-2-Globulin | 4-10 ×10−3 | ||
| beta globulin | 5-12 ×10−3 | ||
| gamma globulin | 6-17 ×10−3 | ||
| Glucagon | range | 5-15 ×10−11 | |
| mean | 7.1-7.9 ×10−11 | ||
| Glucosamine | fetus | 4-6 ×10−4 | 4.2-5.5 ×10−4 |
| child | 5-7 ×10−4 | 5.2-6.9 ×10−4 | |
| adult | 6-8 ×10−4 | 6.1-8.2 ×10−4 | |
| aged | 7-9 ×10−4 | 7.0-8.9 ×10−4 | |
| Glucose | newborn | 2-3 ×10−4 | |
| adult | 6.5-9.5 ×10−4 | 7-10.5 ×10−4 | |
| diabetic | 14-120 ×10−4 | ||
| Glucuronic acid | 4.1-9.3 ×10−5 | 8-11 ×10−6 | |
| Glutamic acid | 2-28 ×10−6 | ||
| Glutamine | 4.6-10.6 ×10−5 | ||
| Glutathione | reduced | 2.5-4.1 ×10−4 | 0 |
| Glycerol | free | 2.9-17.2 ×10−6[2] | |
| Glycine | 1.7-2.3 ×10−5 | 8-54 ×10−6 | |
| Glycogen | 1.2-16.2 ×10−5 | 0 | |
| Glycoprotein, acid | 4-15 ×10−4 | ||
| cGMP | 0.6-4.4 ×10−9 | ||
| Gonadotropin-releasing hormone | 1-80 ×10−12 | ||
| Guanidine | 1.8-2.3 ×10−6 | ||
| Haptoglobin | 3-22 ×10−4 | ||
| Hemoglobin | 1.2-1.75 ×10−1 | 1-4 ×10−5 | |
| newborn | 1.65-1.95 ×10−1 | ||
| children, varies with age | 1.12-1.65 ×10−1 | ||
| adult, male | 1.4-1.8 ×10−1 | ||
| adult, female | 1.2-1.6 ×10−1 | ||
| inside erythrocyte | ~3.3 ×10−1 | ||
| per red blood cell | 27-32 picograms | ||
| Hexosephosphate P | 1.4-5 ×10−5 | 0-2 ×10−6 | |
| Histamine | 6.7-8.6 ×10−8 | ||
| Histidine | 9-17 ×10−6 | 1.1-3.8 ×10−5 | |
| Hydrogen ion(pH 7.4) | 4 ×10−11 | ||
| beta-Hydroxybutyric acid | 1-6 ×10−6 | 1-9 ×10−6 | |
| 17α-Hydroxycorticosteroids | 4-10 ×10−8 | ||
| 17α-Hydroxyprogesterone | male | 20-250 ×10−11 | |
| female, follicular | 20-80 ×10−11 | ||
| female, luteal | 80-300 ×10−11 | ||
| female, postmenopausal | 4-50 ×10−11 | ||
| female, child | 20-140 ×10−11 | ||
| Antibodies | Immunoglobulin A (IgA) | 5-39 ×10−4 | |
| Immunoglobulin D (IgD) | 0.5-8.0 ×10−5 | ||
| Immunoglobulin G (IgG) | 5.0-19 ×10−3 | ||
| Immunoglobulin M (IgM) | 3.0-30 ×10−4 | ||
| Immunoglobulin E (IgE) | <5 ×10−7 | ||
| Indican | 8-50 ×10−7 | ||
| Inositol | 3-7 ×10−6 | ||
| Insulin | 2.0-8.4 ×10−10 | ||
| Insulin-like growth factor | 9.9-50 ×10−8 | ||
| Iodine | total | 2.4-3.2 ×10−8 | 4.5-14.5 ×10−8 |
| Iron | adult | 4-6 ×10−4 | 6-18 ×10−7 |
| Isoleucine | 9-15 ×10−6 | 1.2-4.2 ×10−5 | |
| Ketone bodies | 2.3-10 ×10−6 | 1.5-30 ×10−6 | |
| alpha-Ketonic acids | adult | 1-30 ×10−6 | |
| L-Lactate | arterial | <11.3 ×10−5 | 4.5-14.4 ×10−5 |
| venous | 8.1-15.3 ×10−5 | 4.5-19.8 ×10−5 | |
| Lead | normal | 1-5 ×10−7 | 1-7.8 ×10−8 |
| toxic | >6-10 ×10−7 | ||
| Lecithin | 1.1-1.2 ×10−3 | 1-2.25 ×10−3 | |
| Leptin | 1.2 ×10−8 | ||
| Leucine | 1.4-2 ×10−5 | 1.2-5.2 ×10−5 | |
| Leukocytes (#/cm³) Total: | total, birth | 9.0-30.0 ×106 | |
| total, pediatric | 4.5-15.5 ×106 | ||
| total, adult, range | 4.3-11.0 ×106 | ||
| total, adult, median | 7.0 ×106 | ||
| Neutrophil granulocytes, birth | 6.0-26.0 ×106 | ||
| Neutrophils, pediatric | 1.5-8.5 ×106 | ||
| Neutrophils, adult, range | 1.83-7.25 ×106 | ||
| Neutrophils, adult, median | 3.65 ×106 | ||
| Eosinophil granulocytes birth | 0.4 ×106 | ||
| Eosinophils, pediatric | 0.2-0.3 ×106 | ||
| Eosinophils, adult, range | 0.05-0.7 ×106 | ||
| Eosinophils, adult, median | 0.15 ×106 | ||
| Basophil granulocytes, adult, range | 0.015-0.15 ×106 | ||
| Basophils, adult, median | 0.03 ×106 | ||
| Lymphocytes, birth | 2-11 ×106 | ||
| Lymphocyte, pediatric | 1.5-8.0 ×106 | ||
| Lymphocyte, adult, range | 1.5-4.0 ×106 | ||
| Lymphocyte, adult, median | 2.5 ×106 | ||
| Monocytes, birth, range | 0.4-3.1 ×106 | ||
| Monocytes, birth, median | 1.05 ×106 | ||
| Monocytes, pediatric | 0.4 ×106 | ||
| Monocytes, adult, range | 0.21-1.05 ×106 | ||
| Monocytes, adult, median | 0.43 ×106 | ||
| Phagocytes, birth, range | 6-26 ×106 | ||
| Phagocytes, birth, median | 11 ×106 | ||
| Phagocytes, pediatric, range | 1.5-8.5 ×106 | ||
| Phagocytes, pediatric, median | 4.1 ×106 | ||
| Phagocytes, adult, range | 3.5-9.2 ×106 | ||
| Phagocytes, CD4 cell count | 0.5-1.5 ×106 | ||
| Lipase P | 1.2-1.4 ×10−4 | ||
| Lipids | total | 4.45-6.1 ×10−3 | 4-8.5 ×10−3 |
| Lipoprotein (Sr 12-20) | 1-10 ×10−4 | ||
| Lithium | 1.5-2.5 ×10−8 | ||
| Lysine | 1.3-3 ×10−5 | 2-5.8 ×10−5 | |
| Lysozyme (muramidase) | 1-15 ×10−6 | ||
| alpha 2-macroglobulin | pediatric | 2-7 ×10−3 | |
| male, adult | 0.9-4.0 ×10−3 | ||
| female, adult | 1.2-5.4 ×10−3 | ||
| Magnesium | 3.2-5.5 ×10−5 | 1.8-3.6 ×10−5 | |
| Malic acid | 4.6 ×10−6 | 1-9 ×10−6 | |
| Manganese | 0-2.5 ×10−7 | 0-1.9 ×10−7 | |
| Melatonin | Day | 1.35-1.45 ×10−11 | |
| Night | 6.07-7.13 ×10−11 | ||
| Mercury | normal | <1 ×10−8 | |
| chronic | >20 ×10−8 | ||
| Methemoglobin | 4-6 ×10−4 | ||
| Methionine | 4-6 ×10−6 | 1-15 ×10−6 | |
| Methyl guanidine | 2-3 ×10−6 | ||
| beta-2-microglobulin | 8-24 ×10−7 | ||
| MIP-1a | 2.3 ×10−11 | ||
| MIP-1b | 9 ×10−11 | ||
| Mucopolysaccharides | 1.75-2.25 ×10−3 | ||
| Mucoproteins | 8.65-9.6 ×10−4 | ||
| Nerve growth factor (NGF) | 6-10 ×10−9 | ||
| Niacin | 5-8 ×10−6 | 2-15 ×10−7 | |
| Nitrogen | respiratory gas | 8.2 ×10−6 | 9.7 ×10−6 |
| total, nonrespiratory | 3-3.7 ×10−2 | ||
| Norepinephrine | Neurotransmitter of the sympathetic nervous system | ||
| after 15 min rest | 2.15-4.75 ×10−10 | ||
| when emitted | 8.1 ×10−9 | 8.5 ×10−9 | |
| Nucleotide | total | 3.1-5.2 ×10−4 | |
| Ornithine | 4-14 ×10−6 | ||
| Oxalate | 1-2.4 ×10−6 | ||
| Oxygen (respiratory gas) | arterial | 2.4-3.2 ×10−4 | 3.9 ×10−6 |
| venous | 1.6-2.3 ×10−4 | 1.6 ×10−6 | |
| Oxytocin | male | 2 ×10−12 | |
| female, nonlactating | 2 ×10−12 | ||
| female, pregnant 33-40 wks | 32-48 ×10−12 | ||
| Pancreatic polypeptide | 5-20 ×10−11 | ||
| Pantothenic acid (vitamin B5) | 1.5-4.5 ×10−7 | 6-35 ×10−8 | |
| Para-aminobenzoic acid | 3-4 ×10−8 | ||
| Parathyroid hormone (PTH) | 2-4 ×10−10 | ||
| Pentose | phosphorated | 2-2.3 ×10−5 | |
| Phenol | free | 7-10 ×10−7 | |
| Phenylalanine | 8-12 ×10−6 | 1.1-4 ×10−5 | |
| Phospholipid | 2.25-2.85 ×10−3 | 5-12 ×10−5 | |
| Phosphatase | acid, prostatic | <3 ×10−9 | |
| Phosphorus | inorganic, adult | 2-3.9 ×10−5 | 2.3-4.5 ×10−5 |
| inorganic, children | 4.0-7.0 ×10−5 | ||
| total | 3.5-4.3 ×10−4 | 1-1.5 ×10−4 | |
| Phytanic acid | <3 ×10−6 | ||
| Platelets (#/cm³): | range | 1.4-4.4 ×108 | |
| median | 2.5 ×108 | ||
| Platelet-derived growth factor | 5.0 ×10−8 | ||
| Polysaccharides | total | 7.3-13.1 ×10−4 | |
| Potassium | 1.6-2.4 ×10−3 | 1.4-2.2 ×10−4 | |
| Pregnenolone | 3-20 ×10−10 | ||
| Progesterone (female) | female, follicular | 0.4-0.9 ×10−9 | |
| female, midluteal | 7.7-12.1 ×10−9 | ||
| pregnancy, weeks 16-18 | 30-66 ×10−9 | ||
| pregnancy, weeks 28-30 | 70-126 ×10−9 | ||
| pregnancy, weeks 38-40 | 131-227 ×10−9 | ||
| male | 12-20 ×10−11 | ||
| Proinsulin | fasting | 0.5-5 ×10−10 | |
| mean | 1.42-1.70 ×10−10 | ||
| Prolactin (male) <20 ×10−9 | while awake | 1-7 ×10−9 | |
| during sleep | 9-20 ×10−9 | ||
| Prolactin (female) | follicular | <23 ×10−9 | |
| luteal | 5-40 ×10−9 | ||
| Proline | 1.2-5.7 ×10−5 | ||
| Prostaglandins | PGE | 3.55-4.15 ×10−10 | |
| PGF | 1.26-1.56 ×10−10 | ||
| 15-keto-PGF2a | 5 ×10−10 | ||
| 15-keto-PGE2 | <5 ×10−11 | ||
| Protein | total | 1.9-2.1 ×10−1 | 6.0-8.3 ×10−2 |
| Protoporphyrin | 2.7-6.1 ×10−7 | ||
| Prostate specific antigen | 0-5 ×10−9 | ||
| Pseudoglobulin I | 8-19 ×10−3 | ||
| Pseudoglobulin II | 2-8 ×10−3 | ||
| Purine | total | 9.5-11.5 ×10−5 | |
| Pyrimidine nucleotides | 2.6-4.6 ×10−5 | 2-12 ×10−7 | |
| Pyridoxine (Vitamin B6) | 3.6-90 ×10−9 | ||
| Pyruvic acid | 3-10 ×10−6 | 3-12 ×10−6 | |
| RANTES | 7 ×10−11 | ||
| Relaxin | day <100 preparturition | <2 ×10−9 | |
| day 100 to 2 days preceding | 5-40 ×10−9 | ||
| day preceding parturition | 100-200 ×10−9 | ||
| day following parturition | <2 ×10−9 | ||
| Retinol (Vitamin A) | 1-8 ×10−7 | ||
| Riboflavin (Vitamin B2) | 1.5-6 ×10−7 | 2.6-3.7 ×10−8 | |
| RNA | 5-8 ×10−4 | 4-6 ×10−5 | |
| Secretin | 2.9-4.5 ×10−11 | ||
| Serine | 3-20 ×10−6 | ||
| Serotonin (5-hydroxytryptamine) | 1.55-1.81 ×10−7 | 0.8-2.1 ×10−7 | |
| Silicon | 1.4-2.95 ×10−6 | 2.2-5.7 ×10−6 | |
| Sodium | 3.1-3.4 ×10−3 | ||
| Solids, total | 2-2.5 ×10−1 | 8-9 ×10−2 | |
| Somatotropin | growth hormone | 4-140 ×10−10 | |
| Sphingomyelin | 1.5-1.85 ×10−3 | 1-4 ×10−4 | |
| Succinic acid | 5 ×10−6 | ||
| Sugar, total | 7-11 ×10−4 | ||
| Sulfates | inorganic | 8-12 ×10−6 | |
| Sulfur | total | 3.8-5 ×10−2 | 3.1-3.8 ×10−2 |
| Taurine | 3-21 ×10−6 | ||
| Testosterone (male) | free | 5.6-10.2 ×10−11 | |
| total | 275-875 ×10−11 | ||
| Testosterone (female) | free | 0.24-0.38 ×10−11 | |
| total | 23-75 ×10−11 | ||
| pregnant | 38-190 ×10−11 | ||
| Thiamine (Vitamin B1) | 3-10 ×10−8 | 1-9 ×10−8 | |
| Thiocyanate | 5-14 ×10−6 | ||
| nonsmoker | 1-4 ×10−6 | ||
| smoker | 3-12 ×10−6 | ||
| Threonine | 1.3-2 ×10−5 | 0.9-3.2 ×10−5 | |
| Thyroglobulin (Tg) | <5 ×10−8 | ||
| Thyroid hormones | 4-8 ×10−8 | ||
| Thyrotropin-releasing hormone | 5-60 ×10−12 | ||
| Thyroxine (FT4) | free | 8-24 ×10−12 | |
| total | 4-12 ×10−8 | ||
| Thyroxine-binding prealbumin | 2.8-3.5 ×10−4 | ||
| Thyroxine-binding globulin | 1.0-3.4 ×10−7 | ||
| Tin | 0-4 ×10−7 | 0-1 ×10−7 | |
| alpha-Tocopherol (Vitamin E) | 5-20 ×10−6 | ||
| Transcortin | male | 1.5-2 ×10−5 | |
| female | 1.6-2.5 ×10−5 | ||
| Transferrin | newborn | 1.3-2.75 ×10−3 | |
| adult | 2.2-4 ×10−3 | ||
| age >60 yrs | 1.8-3.8 ×10−3 | ||
| Triglycerides | 8.5-23.5 ×10−4 | 2.5-30 ×10−4 | |
| Triiodothyronine | free | 2.3-6.6 ×10−12 | |
| total (T3) | 0.75-2.50 ×10−9 | ||
| Tryptophan | 5-10 ×10−6 | 9-30 ×10−6 | |
| Tyrosine | 8-14 ×10−6 | 4-25 ×10−6 | |
| Urea | 2-4 ×10−4 | 1.5-4.7 ×10−4[1] | |
| Uric acid | child | 2.0-6.7 ×10−5[3] | |
| adult, male | 3.4-7.2 ×10−5[1] | ||
| adult, female | 2.4-6.1 ×10−5[1] | ||
| Valine | 2-2.9 ×10−5 | 1.7-4.2 ×10−5 | |
| Vasoactive intestinal peptide (VIP) | 6-16 ×10−12 | ||
| Vasopressin | hydrated | 4.5 ×10−13 | |
| dehydrated | 3.7 ×10−12 | ||
| Zinc | 5-13 ×10−6 | 7-15 ×10−7 | |
In blood banking, the fractions of Whole Blood used for transfusion are also called components.
Leave a Reply
You must be logged in to post a comment.